Mr. kousen is … Water Man.
Water Man.
BIOCHEMISTRY
The
Chemistry of Living Things
Hi. Thanks
for stoppin' by. This area of my "biology help pages" is about biochemistry,
an area that many students find pretty challenging (difficult). While the
ideas are abstract, much of the material boils down to memorization.
Memorization boils down to studying. Studying boils down to work. Work
boils down to effort. So, put your best effort forward & let's
get to work !
Organic
vs Inorganic compounds:
"All living
things are composed of one or more cells and the products of those cells."
Now where
have you seen that before ? That is 1/3 of the cell theory, right ? The
chemical compounds that make up the structures in cells are a mixture of
organic compounds and inorganic compounds. To keep it simple, remember
it this way : organic compounds always contain carbon and hydrogen
(and maybe some other elements), inorganic compounds do not contain carbon
and hydrogen together.
Organic compounds
are found in living things, their wastes, and their remains.
Examples
of organic compounds : carbohydrates (sugars, starches), lipids (fats &
waxes), proteins, nucleic acids (DNA & RNA).
Examples
of inorganic compounds : water, carbon dioxide.
The elements
(atoms) in organic compounds are held together by covalent bonds, which
form as a result of the sharing of two electrons between two atoms.
For now, let's
save any other nitty-gritty chemistry details for chemistry, OK ?
Chemical
Formulas :
There are
three kinds of chemical formulas we should understand. The simplest
is the "molecular formula", which tells you the number of atoms of each
element present in a compound. An "empirical formula" is basically a molecular
formula with the numbers of atoms shown in the smallest possible ratio.
A structural formula is like a diagram of the compound. It shows
the atoms present and how they are arranged and bonded together in the
compound.
Here are the
molecular, empirical, & structural formulas for one compound that we
will all learn to love --- GLUCOSE.
CHEMICAL FORMULAS
FOR GLUCOSE
|
Molecular
Formula
|
Empirical
Formula
|
Structural
Formula
|
|
C6H12O6
|
CH2O
|
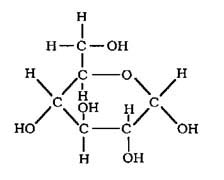 |
Glucose is an example of
a "monosaccharide", a small carbohydrate.
-
The molecular
formula tells us that there are 6 carbon atoms, 12 hydrogen atoms, &
6 oxygen atoms in one single glucose molecule.
-
Notice that if
you look at the structural formula & tally up each letter (element)
you get the molecular formula.
-
Each line (dash)
represents the covalent bond holding the atoms together.
-
The ratio of
the elements in the molecular formula is 6:12:6, which reduces to 1:2:1
(the number expressed in the empirical formula : CH2O --- we
don't bother writing the "1"s).
Understanding
the formulas is very important --- they are like vocabulary in this chapter,
if you don't know 'em, it'll be like trying to read & understand this
: capt bio for president
!
Dehydration
Synthesis vs Hydrolysis :
All of the organic compounds we
will study are examples of polymers. A polymer is a large chemical
compound composed of smaller repeating units --- over & over &
over again. Like a long choo-choo train is made up of smaller connected,
repeating, choo-choo cars.
The chemical process that connectsthe
smaller subunits to form large organic compounds is called dehydration
synthesis. Remember "synthesis" from chapter 1 ? It still means
the same thing : build. The "dehydration" part of the term refers to the
fact that water is lost during the chemical process that bonds the subunits
together. We will "see" this in a minute when we get more specific.
Hydrolysis is the process
that breaks large organic compounds
into their smaller subunits. It is the opposite of dehydration synthesis.
In HYDROlysis, water (hydro) is added and the large compounds are split
("lysis" means split). The process of hydrolysis is involved in digestion
--- when food is broken down into nutrients.
So, to summarize :
| PROCESS |
STARTS WITH ... |
ENDS WITH ... |
EXAMPLE |
| dehydration synthesis |
small molecules
(subunits) |
large molecules & water |
|
| hydrolysis |
water &
large molecules |
small molecules
(subunits) |
digestion |
You will do yourself
a BIG favor if you can keep these two processes straight.
QUESTIONS
- Organic Compounds, Formulas, Dehydration Synthesis & Hydrolysis
Before we get into specific kinds
of organic compounds, let's try some questions about what we've done so
far.
1. Which is an example
of an organic compound ?
a)C17H35COOH
b) (NH4)3PO4 c) H2O d) NaCl
2. Which type of formula
gives the most information about a compound ?
a) molecular
b) empirical c) structural
3. How many atoms of
hydrogen in C12H22O11 ?
4. Identify three
inorganic compounds in the following reaction :
CO2
+ H2O + sunlight ---> C6H12O6
+ O2
5. Is the following reaction
an example of dehydration synthesis or hydrolysis ? How do you know ?
C12H22O11
+
H2O ---> C6H12O6 + C6H12O6
<check
your answers
CARBOHYDRATES:
| Elements
Present |
Used by organisms
for ... |
Building
Block |
Related Terms
& Info |
carbon
hydrogen
oxygen
H:O = 2:1
always ! |
energy
structure |
monosaccharides
(simple sugars)
ex: glucose |
dissaccharide = 2 connected
monosaccarides
(ex: maltose)
polysaccharide
3 or more connected monosaccarides
(ex: starch, glycogen, chitin, cellulose)
|
NOTES:
-
The 2:1 ratio of hydrogen to oxygen
atoms in all carbohydrates is a very important identifying characteristic.
-
Another clue to identifying carbohydrates
is their structure. Monosaccharides have a ring-like structure, kind of
like a hexagon. So if you are looking at structural formulas and you see
"rings", it's probably a carbohydrate; especially if only carbon, hydrogen,
& oxygen are present in the molecule. Want to see what I mean ?

-
The ring-thing is a big deal. It will
help you. Memorize it.
-
What we have in the equation above
is two single rings (monosaccharides) on the left becoming chemically combined
to form the two-ringed molecule on the right (a disaccharide). It is a
synthesis reaction --- the product is bigger than the individual reactants.
-
In order to combine the two glucose
molecules, bonds must become available. This is accomplished by removing
a hydrogen ion (H+) from one glucose & a hydroxyl ion (OH-)
from the other (the dashed box in the equation illustrates this point).
These ions bond to form the water molecule that appears on the far right.
This happens in every dehydration synthesis reaction --- water is
lost as a waste product.
-
If we were to turn the arrow in the
equation around & read from right to left, we would be looking at the
HYDROLYSIS
of maltose. In the hydrolysis of maltose, water would be added to the disaccharide
(maltose) causing it to split into its smaller subunits --- the
two monosaccharides (glucose molecules).
-
Not to beat a dead horse, but the fact
that only C, H, & O are in the molecules, and that the molecules have
a ring-like structure should make you very confident in identifying them
as carbohydrates.
-
Getting back to the carbohydrate table,
chitin and cellulose are examples of carbohydrates with structural functions.
Chitin is the material that makes up the exoskeletons of all arthropods
(insects, spiders, lobsters, etc.). Cellulose is what the cell wall in
plant cells is made of.
-
Starch is the form by which plants
store extra carbohydrates. Glycogen (sometimes referred to as "animal
starch") is the form by which animals store extra carbohydrates.
We store glycogen in our livers.
PROTEINS:
Allow me to make my first point
about proteiNs by writing "proteiN" like this : proteiN.
"N" stands for nitrogen. There is an "N" in the word proteiN.
The element nitrogen is always present in proteiNs. This will help you.
Memorize it. :)
| Elements Present |
Used by organisms for ... |
Related Terms & Info |
carbon
hydrogen
oxygen
NITROGEN
(always those 4)
phosphorus
sulfer
(possibly)
|
structure & movement (muscles)
enzymes
antibodies
hormones
pigments |
peptide bond = the bond that
holds amino acids together in protein molecules
dipeptide = two connected amino
acids
polypeptide = 3 or more connected
amino acids
|
Building Block
of Proteins:
|
amino acids
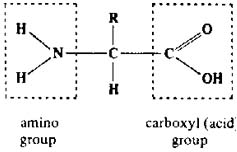
|
-
Well, where to start. Did you
notice the "N" in the amiNo group ? Since big proteiN molecules (which
we call polypeptides) are long chains of amino acids, every (every) proteiN
has nitrogen in it. Always.
-
You are responsible for recognizing
& identifying the smaller parts of an amino acid. The NH2
on the left is the amino group, the COOH on the right is called a carboxyl
group. The carboxyl group is responsible for giving the amino acids its
"acid" properties.
-
The "R" is not an individual atom or
element. Instead, the "R" spot is the location at which one of a number
of groups of atoms connect to the rest of the amino acid. They are called
"variable groups". There are 20 different variable groups --- so there
are 20 different amino acids. So what I am trying to say is that
the basic structure of all amino acids is the same except for the variable
group ("R") spot. And whichever of the 20 variable groups you have
bonded there determines which of the 20 amino acids you're dealing with.
Let me illustrate with an example:
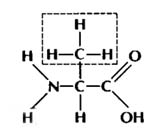
alanine
"R" group = CH3
|
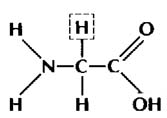
glycine
"R" group = H
|
-
Both of these are amino acids because
they have an amino group (NH2) on the left & a carboxyl
group (COOH) on the right. They are two different amino acids because they
have different atoms bonded at the "R" group spot. See ? That's not
so bad, is it ?
-
Now, tell me something. By what process
are individual amino acids combined to from larger proteiNs ? Very very
good ... dehydration synthesis. This is THE process by which ANY small
organic molecules are combined to form BIG organic molecules. The dehydration
synthesis of a protein is typically illustrated like so:
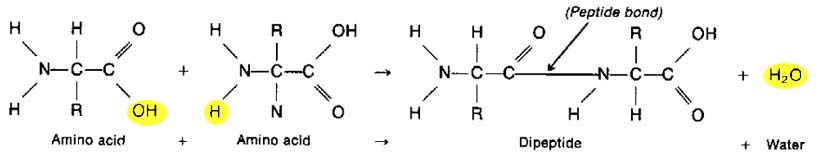
-
There are two clues that what you are
looking at in the above equation is dehydration synthesis. The first is
that water is at the end --- a waste product in this process ("dehydration"
= loss of water !). The 2nd clue is that the one molecule on the
right (the dipeptide) is bigger than the individual reactants (amino acids)
on the left (synthesis = build).
-
Now, just like with putting 2 monosaccarides
together, we can't combine the two amino acids until we have freed some
bonds up. This is accomplished by removing an OH from one amino acid
& an H from the other. These atoms bond & live happily ever after
as H2O (water). The yellow in the diagram above is my attempt
to emphasize this idear. The removal of OH's & H's & the formation
of water as a waste product happens in EVERY dehydration synthesis reaction
--- whether it involves carbohydrates, proteins, or lipids.
-
Notice please that the bonds "freed
up" after the removal of water form the "peptide bond".
-
"Dipeptide" is just a word for two
amino acids that are bonded together. If we continued to add more &
more amino acids to the dipeptide we would then call the molecule a POLYpeptide.
-
If you haven't noticed already, "peptide"
is a protein word. Dipeptide, polypeptide, peptide bond, --- all protein
stuff.
-
The hydrolysis (breakdown) of a dipeptide
could be summarized like this:
dipeptide + water ---> amino
acid + amino acid
Notice that water is added
at the beginning in hydrolysis, & that our products are smaller
than the molecule we start with.
i THinK THat
wE'vE TRied to STUFF eNOUGh inTo yOur BRAIN for nOW. WE'd bETTEr maKe surE
SoMe STuFF is STICkiNG. InterESTeD
IN a quiz?
it'S on carbOhyDRAtes & prOTEiNs. C'mon, give it a shot.
Elements
Present
|
Used
by Organisms for ...
|
Related
Terms & Info
|
Carbon
Hydrogen
Oxygen
ONLY !
There is no specific H:O ratio. |
Stored Energy
Structure
(important part of cell membranes) |
saturated fat = C-C bonds
are all single bonds
unsaturated fat = contain
at least one double or triple C-C bond |
Building Blocks
of Lipids |
fatty acid :
glycerol : 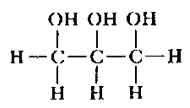
|
-
-
-
-

-
Like other dehydration synthesis reactions,
we must free some bonds before we combine the 3 fatty acids & glycerol.
And like before, this is accomplished by removing water molecules.
We remove 3 waters in this reaction because we are bonding 3 fatty acids
to the glycerol (we need 3 free bonds).
-
Notice that there is no Nitrogen anywhere,
so this is definately not a proteiN reaction.
-
Notice also that there are no
ring-shaped molecules, so we are not dealing with carbohydrates either.
-
The hydrolysis (digestion) of a lipid
could be summarized like so:
lipid + water ----> 3 fatty
acids + glycerol
NUCLEIC
ACIDS: DNA & RNA
We will save the nitty gritty details
of DNA & RNA for later in the year (Genetics). But for now, you
should know there functions & basic structure, and how DNA compares
to RNA.
-
DNA & RNA (like proteins, carbohydrates,
& lipids) are polymers --- long chains of smaller repeating units.
The repeating unit in nucleic acids is called a nucleotide.
-
Every nucleotide has the same basic
structure:
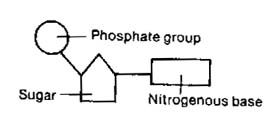
-
the phosphate is a PO4
-
the sugar (see the ring?) has 5 carbons
(one at each corner)
-
the N-base is one of four possibilities
(more on that in a second ...)
COMPARISON
OF DNA & RNA
|
DNA
|
RNA
|
|
FULL NAME
|
Deoxyribonucleic acid
|
Ribonucleic acid
|
|
BASIC
STRUCTURE
|
2 long twisting strands of nucleotides in the form af a "double
helix"
|
1 single strand of nucleotides
|
|
NUCLEOTIDE SUGAR
|
Deoxyribose
|
Ribose
|
| NITROGENOUS BASES |
guanine (G)
cytosine (C)
adenine (A)
thymine (T) |
guanine (G)
cytosine (C)
adenine (A)
uracil (U) |
| LOCATION IN A CELL |
nucleus
(the chromosomes) |
nucleus, in the cytoplasm, & at the ribosomes |
| FUNCTION |
the hereditary material
of a cell, directs & controls cell activities |
involved in protein synthesis |
-
so DNA & RNA are alike in that
they are both nucleic acids composed of nucleotides
-
their differences lie in their funcstions
and structure
-
the main structural differences are
the number of strands in the molecule, the sugar structure, and one of
the N-bases (thymine in DNA, uracil in RNA)
 Well, congratulations
for getting through all this stuff. It is by far one of the two hardest
topics in the biology syllabus. I hope this stuff helped. Keep pluggin'
away.
Well, congratulations
for getting through all this stuff. It is by far one of the two hardest
topics in the biology syllabus. I hope this stuff helped. Keep pluggin'
away.
Back to Biology Topics Outline

IF YOU HAVE COMMENTS (GOOD
OR BAD) ABOUT THIS OR ANY OF MY BIOLOGY PAGES,CLASSES OR ANYTHING ELSE
IN GENERAL, DROP ME A NOTE:
kousenclass@hobart.k12.in.us
ANSWERS
: THE CHEMISTRY OF LIVING THINGS
QUESTIONS :Organic
Comp., Formulas, Dehydration Synth. & Hydrolysis
Answers
& explanations are in black.
1. Which is an example
of an organic compound ?
a)C17H35COOH
b) (NH4)3PO4 c) H2O d) NaCl
*
Organic compounds must have both carbon (C) & hydrogen (H) in
them.
2. Which type of formula
gives the most information about a compound ?
a) molecular
b) empirical c) structural
*
the structural give you number & types of atoms & their arrangement
3. How many atoms of
hydrogen in C12H22O11 ? 22
(the # after the H)
4. Identify three
inorganic compounds in the following reaction :
CO2
+ H2O + sunlight ---> C6H12O6
+ O2
*
Organic compounds must have both C & H in them. Anything else
is inorganic.
5. Is the following reaction
an example of dehydration synthesis or hydrolysis ? How do you know ?
C12H22O11
+
H2O ---> C6H12O6 + C6H12O6
*
hydrolysis. we know for two reasons : 1) the two molecules we end
up with (on the right) are smaller than the one on the left; & 2) water
is added
<back
to questions









