 |
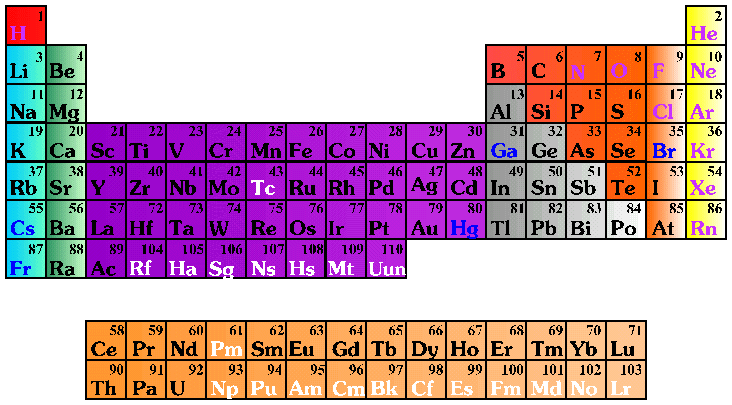
In 1869 Dmitri Mendeleev developed the original periodic table which has changed over the years, but the basic idea remains the same. He left three gaps which were later filled in with gallium, scandium, and germanium. Originally Mendeleev organized the elements according to their atomic mass. However, Henry Moseley noticed that if the elements were arranged according to their number of protons or electrons a pattern developed and it became clear how many elements actually remained unknown. It is Moseley's periodic table that we use today. The elements are numbered according to the number of their protons and electrons. An example is hydrogen. It has one proton and one electron. Its number on the periodic table is one. Another example is gold. It has seventy-nine protons and seventy-nine electrons. Its number on the periodic table is seventy-nine.
The rows of the table are called periods. A period is a group of elements whose properties are basically the same and their gradual changes can be predicted.
The columns of the table are called groups. A group consists of elements that have similar chemical or physical properties.
The table can be divided in several different ways. Some will divide it among these four categories:
| Main Group Elements
|
which include the elements in the two columns on the furthest left ~ hydrogen, lithium, beryllium, sodium, magnesium, potassium, calcium, rubidium, strontium, cesium, barium, francium, and radium) and the elements in the six columns on the furthest right (helium, boron, carbon, nitrogen, oxygen, fluorine, neon, aluminum, silicon, phosphorus, sulfur, chlorine, argon, gallium, germanium, arsenic, selenium, bromine, krypton, indium, tin, antimony, tellurium, iodine, xenon, thallium, lead, bismuth, polonium, astatine, and radon (Groups 1, 2, and 13 - 18) |
Transition Metals
|
which connect the two sides of the table represented by the main group elements (Groups 3 - 12) |
| Lanthanides | which are part of the rare earth metals shown in the last two rows of the periodic table, known as the transition metals because they represent the elements missing from the main table |
Actinides |
which are part of the rare earth metals shown in the last two rows of the periodic table, known as the transition elements because they represent the elements missing from the main table |
Others divide it up among the different families represented by the elements:
|
|
Very rarely, they are grouped together according to their natural phase at room temperature:
Gas, Liquid, Solid or Synthetic?
| Elements by Name | Elements by Number | Elements by Energy Levels | Home |